Comparison between Isothermal and Adiabatic Process
Comparison between Isothermal and Adiabatic Process: Overview
This topic covers concepts, such as, Isotherm, Slope of Isotherm, Comparison between Isothermal and Adiabatic Indicator Diagrams & Limitations of First Law of Thermodynamics etc.
Important Questions on Comparison between Isothermal and Adiabatic Process
Can two isothermal curves cut each other?
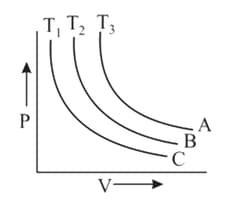
The PV diagram for three different isothermal processes A, B and C are shown above. Chose the correct option.
What is the equation of entropy?
Which among this is not an exothermic reaction?
The equation for the average kinetic energy is ________
A ideal gas at is compressed adiabatically to of its original volume. The rise in temperature is ( Take )
A diatomic ideal gas is compressed adiabatically to of its initial volume of the gas is (in Kelvin) and the final temperature is , the value of is
The slope of isothermal and adiabatic curves are related as
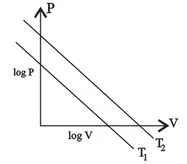
For the following two isotherms drawn at two different temperature and which of the following statement is true? ( number of moles are constant )
An ideal gas at is compressed adiabatically to of its original volume. The rise in temperature is (Take )
The slope of isothermal and adiabatic curves are related as
Which of the following is isentropic process
In an isothermal process if heat is released from an ideal gas then
The graph for Boyle's law is called
Which is the correct graphical representation for ideal gas under isothermal condition?
Which of the following statements about the diagram of an ideal gas of fixed number of particles is incorrect?
From the given options, using which variables for X and Y -axis we can make an isotherm graph?
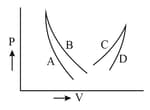
P-V plots for two gases during adiabatic processes are shown in the figure. Plots and should correspond respectively to:
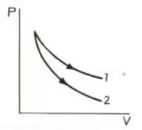
In adjoining diagram, no heat exchange between the gas and the surroundings will take place if the gas is taken along:
An ideal gas expands isothermally from a volume to and then compressed to original volume adiabatically. The initial pressure is and final pressure is . The total work done is . Then
